Kodai Hirano, Yuta Nonami, Yoshiaki Nakamura, Toshiyuki Sato, Takuya Sato, Keiichiro Ishiguro, Takehiko Ogawa, *Shosei Yoshida
Background
Spermatogenesis in mammals is a temperature-sensitive developmental process that occurs at a few degrees below the core body temperature of 38 °C. Elevated testis temperatures disrupt spermatogenesis at various phases, leading to male infertility. Artificial cryptorchidism, which involves surgically translocating the testis into the abdominal cavity, is a commonly used method to study the effect of temperature on spermatogenesis. However, this approach has limitations because of variations in surgical technique, inability to accurately control testicular temperature, and the inability to exclude influences of extra-testicular influencers such as hormones.
To overcome these limitations, the authors of a recent study have used an air-liquid interface (ALI) organotypic culture of whole mouse testicular explants, which can support complete spermatogenesis over several weeks and lead to the production of functional sperm (Sato et al., 2011). This culture setup enables the investigation of the chronic effect of precisely controlled temperatures on mouse spermatogenesis under conditions free from extratesticular factors.
Key findings:
1) At 38, 37 and 36°C, spermatogenesis does not proceed beyond early spermatocytes, late spermatocytes and round spermatids, respectively
Whole testes from P4 neonatal Acr-GFP mice were cultured for 5 weeks at the ALI at 30°C, 32°C, 34°C (scrotal temperature), 35°C, 36°C, 37°C, 38°C, 39°C or 40°C. Non-invasive analysis of GFP (expression starts in pachytene spermatocytes) was performed throughout the whole culture period. Additionally, 5-week cultured testicular sections were subjected to immunofluorescence for MVH (pan-germ cells), SCP3 (spermatocytes), KIT (differentiating spermatogonia) and GFRα1 (a fraction of undifferentiated germ cells). The authors discovered that the optimal temperature for spermatogenesis was 34 °C, as evidenced by the presence of all stages of germ cell development (Fig. 1). The Acr-GFP signal appeared in the seminiferous tubules from 2 to 5 weeks at 32-37 °C, indicating that germ cells had progressed to the late pachytene stage, and beyond. Despite comparable Acr-GFP signals, the number of elongating spermatids at 32 °C and 35 °C were lower than at 34 °C, and absent at 36 °C. An increase in temperature to 37 °C resulted in a weaker Acr-GFP signal, with Acr-GFP+ spermatocytes being the most advanced cell type. In contrast, the Acr-GFP signal was not detectable throughout the culture period when cultured at 38-40 °C. At 30 °C and 39 °C, germ cell numbers were reduced, and became absent at 40 °C (Fig. 1). Additionally, GATA4+ Sertoli cells were abundantly observed at all temperatures, indicating that temperature sensitivity is a prominent trait of germ cells.
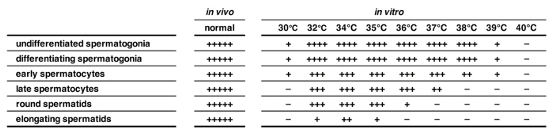
Fig 1. Summary of the qualitative evaluation of spermatogenic cell types observed in testis explants cultured for 5 weeks at the respective temperatures. −, never observed; +, observed infrequently but reproducibly; ++, found easily in some peripheral tubules; +++, observed in most peripheral tubules as a group of cells; ++++, always observed as a robust population close to in vivo samples; +++++, observed as a completely developed population at levels of in vivo-developed testis, which never occurred in our ex vivo cultures
2) High temperature compromises meiotic prophase I and causes apoptosis
The process of meiotic prophase I involves the establishment of synapsis of homologous chromosomes and crossover through the stages of leptotene, zygotene, pachytene, and diplotene. To investigate the underlying mechanism(s) of meiotic failure associated with heat stress and to determine which phase is affected by high temperatures, meiotic chromosomal spreads of spermatocytes from testicular explants cultured at 34 °C, 37 °C, and 38 °C were analyzed by immunofluorescence for SCP3 and SCP1 (axial/lateral elements of the synaptonemal complex) staining. SCP3 and SCP1 consistently localized in spermatocytes at all tested temperatures, indicating that all stages of meiotic prophase I were present. At 34 °C, the proportions of spermatocytes were similar to those in vivo. However, at 37 °C, only a small fraction of spermatocytes progressed to the pachytene and diplotene stages, while at 38 °C, pachytene and diplotene spermatocytes were virtually absent. Furthermore, spermatocytes failed to complete chromosome pairing and eventually underwent apoptosis (Caspase 3+) at 38 °C. Although some spermatocytes survived until the pachytene and diplotene stages at 37 °C, they failed to complete meiotic divisions and develop into spermatids. The findings are consistent with the observation that Acr-GFP signals are undetectable and weak at higher temperatures.
3) More double strand breaks (DSB) occur, in part, due to defective repair machinery because of high temperatures
Staining for γH2AX was used to assess the levels of DNA DSBs at the different temperatures. These were found to increase at 38 °C and, to a lesser extent, 37 °C, as compared to 34 °C. Subsequent to γH2AX analyses, the authors conducted staining for RAD51 and DMC1 (DSB repair proteins) to investigate the formation of recombination foci in spermatocytes at different temperatures. Explants maintained at 34 °C showed foci numbers similar to in vivo, although RAD51 foci numbers were slightly increased. However, at 38 °C, the authors observed reduced numbers of RAD51 and DMC1 foci in late leptotene and zygotene spermatocytes. At 37 °C, foci numbers were slightly reduced in late leptotene and zygotene, and the few pachytene spermatocytes that survived showed similar numbers to the in vivo control. These findings suggest that the recruitment and assembly of RAD51 and DMC1 to form recombination foci are compromised at 37 °C and 38 °C, likely contributing to the observed increase in unresolved DSBs (γH2AX) at elevated temperatures.
4) Abundant DSBs cause asynapsis
Normally, synapsis occurs synchronously in zygotene spermatocytes when both synapsed and unsynapsed regions are present in the nucleus. However, in explants grown at 37 °C and 38 °C, the authors observed disordered chromosome pairing in some zygotene spermatocytes, with some chromosomes completely synapsed and others unsynapsed. They also observed pairing of non-homologous chromosomes, defined as “aberrant pachytenespermatocytes”. Such cells were rare in vivo but more abundant in explants grown at 37 °C and 38 °C. No MLH1 foci (meiotic recombination) could be observed in aberrant pachytene spermatocytes at any temperature tested, suggesting that these cells do not activate the meiotic program that usually occurs in late pachytene. In contrast, fully synapsed pachytene spermatocytes surviving at 34 °C and 37 °C harbored MLH1 foci, indicating their progression to late pachytene. However, the number of MLH1 foci decreased at 37 °C, and chromosome pairs lacking foci were often observed in these pachytene spermatocytes. Therefore, crossover formation was compromised in late pachytene spermatocytes developed ex vivo, less at 34 °C and critically from 37 °C.
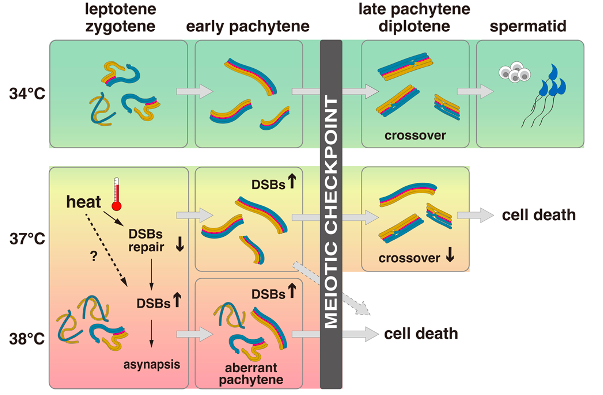
Fig. 2 The effects of temperature on spermatogenesis with a focus on meiosis. At a temperature of 34 °C, meiosis is completed normally and spermatids are formed. However, at higher temperatures of 37 °C or 38 °C, DSBs increase, leading to asynapsis, compromised crossover formation, and cell death without completing meiotic divisions. The degree of impairment varies between cells, with severely affected cells undergoing aberrant pachytene and less-affected cells completing chromosome pairing. The meiotic checkpoint eliminates cells with DSBs and asynapsis above a certain threshold through apoptotic cell death, while cells below the threshold survive until late pachytene. All cells are eliminated at 38 °C, while some cells survive until late pachytene at 37 °C but retain abnormally abundant DSBs and asynapsis.
