During the differentiation phase at the spermiogenesis (the last phase of the spermatogenesis), haploid round spermatids undergo an extreme morphological remodelling of nuclear and cytoplasmic structures. Nonetheless, while testicular sperm are morphologically specialized cells, they are not functional and lack both the ability to swim and fertilize. Hence, sperm maturation in the epididymis is therefore essential to obtain these abilities.
After release of spermatozoa from the Sertoli cell in the seminiferous tubules, the spermatozoa continue their journey through the epididymis; from the caput (proximal part) to the cauda (distal part) epididymis (highlighted in green and blue, respectively, in section A from the figure below). Epididymal transit is accompanied by substantial modifications of protein, lipid, and RNA contents in the transcriptionally and translationally silent sperm cells. These modifications are driven by the exposure of spermatozoa to external factors secreted by the epididymis. In this article, Skerret-Byrne and colleagues describe the most extensive characterization of the mouse sperm proteomic changes associated with epididymal maturation. Their characterization has increased the mouse sperm proteome to more than 6000 proteins, providing protein evidence of over 650 annotated proteins within the mouse genome without previous confirmation at the protein level.
Interestingly, the authors show that epididymal transit is associated with a large-scale modification of the sperm proteome, primarily driven by the loss of more than 50% protein content versus an acquisition of 9% cauda sperm proteins. Additionally, from the approximately 2500 proteins that are maintained during the epididymal maturation, around 900 proteins present altered abundance, which predominantly decreased in sperm from cauda epididymis.
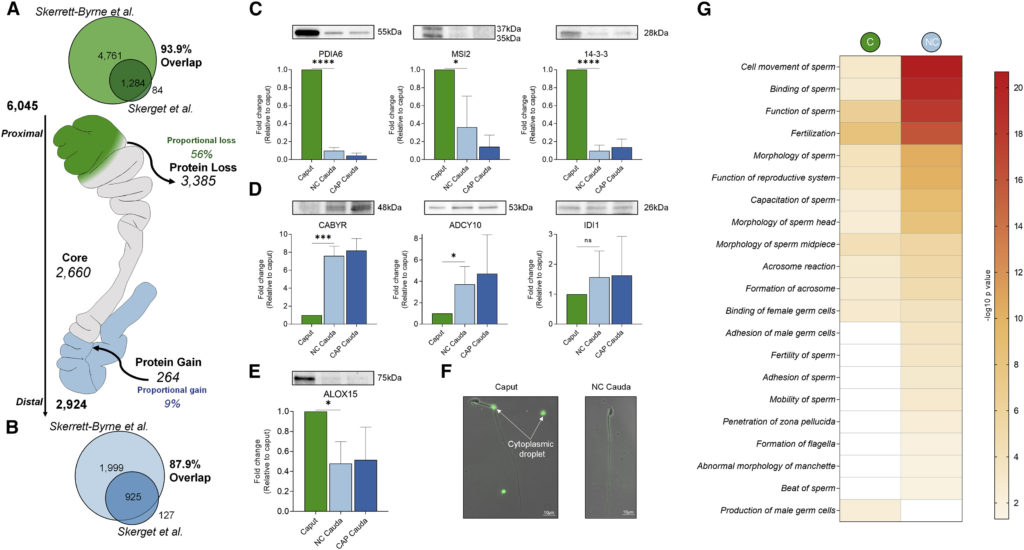
Despite losing over half of their protein content, sperm isolated from the cauda epididymis presented an even more specialized proteome enriched in proteins relevant for male reproduction and sperm function. Likewise, the analysis showed that the lost proteins were associated with male germ cell production, reinforcing the relevance of the epididymis-associated proteomic changes in sperm maturation. Additionally, proteins related with known sperm disorders or infertility phenotypes were lost or underrepresented in cauda spermatozoa. Compared to the subtle protein changes resulting from the subsequent process of sperm capacitation, in which sperm acquire hyperactive motility in the female reproductive tract, this work evidences that the most drastic remodelling of the sperm proteomic profile during post-testicular maturation occurs in the epididymis.
As a mechanistic insight, the authors hypothesize that proteins that are lost during the epididymal sperm maturation could potentially be retained in cytoplasmic droplets. Moreover, several proteins newly acquired during the epididymal transit were expressed in mouse epididymis, suggesting their acquisition via extracellular vesicles secreted by epididymal epithelium (epididymosomes).
Thus, these results help to better understand sperm cell biology and the evolutionary investment of all changes accompanying sperm maturation in the epididymis. Therefore, the consequences of a wrong protein uptake and shed during epididymal transit could equally have detrimental consequences for sperm function. If you are interested in knowing more about this work, do not miss the opportunity to meet Prof. Brett Nixon (corresponding author) in person during the upcoming 15th NYRA Meeting in Caux!

