Sex chromosomes are important for the development of sperm and egg cells in mammals. In mice, reproductive cells start off as the same type of cell and then develop differently depending on the biological niche they are in. In the case of males (XY), primordial germ cells (PGCs) develop into sperm and in females (XX), these develop into oocytes. However, experiments in mice have shown that this process does not always work as biologically expected. For instance, in sex reversal models, XX PGCs cannot differentiate into sperm and XY PGCs produce low-fertility oocytes due to mispairing of sex chromosomes or gene expression issues. A recent study has gone a step further and provided evidence that stem-cell-like induced pluripotent stem cells (iPSCs) derived from XY males can be used to generate oocytes.
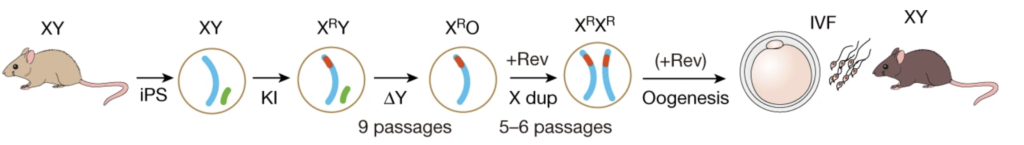
Figure 1. Schematic of the production of biparental offspring.
Murakami and colleagues developed a novel in vitro culture system, involving reprogramming of male PGCs to iPSCs, followed by differentiation into primordial germ cell-like cells (PGCLCs) and aggregation with gonadal somatic cells to promote oogenesis. The team found that the production of functional oocytes from PGCLCs derived from XY cells was severely impaired, even in the presence of female gonadal somatic cells. This was attributed to the delay of meiotic initiation and asynapsis in oocytes with abnormal sex chromosome complements. The results confirmed that appropriate sex chromosomes in both germ cells and somatic cells are essential for proper gametogenesis. Furthermore, the study demonstrated that the removal of the Y chromosome and/or duplication of the X chromosome is essential for oocyte production from cells with sex chromosome disorders. To achieve the latter, the team used CRISPR/Cas9 genome editing to alter the sex chromosome complement in the resulting male PGCLCs. The resulting XY iPSCs without the Y chromosome or with two X chromosomes differentiated into functional oocytes with normal meiotic progression and chromosomal alignment. In addition, those oocytes gave rise to offspring without apparent abnormalities after fertilisation with sperm and implantation into surrogate female mice. Despite efforts of generating both gametes in vitro from the genetically edited iPSC, generation of offspring was not achieved due to the low fertility potential of both gametes produced in vitro.
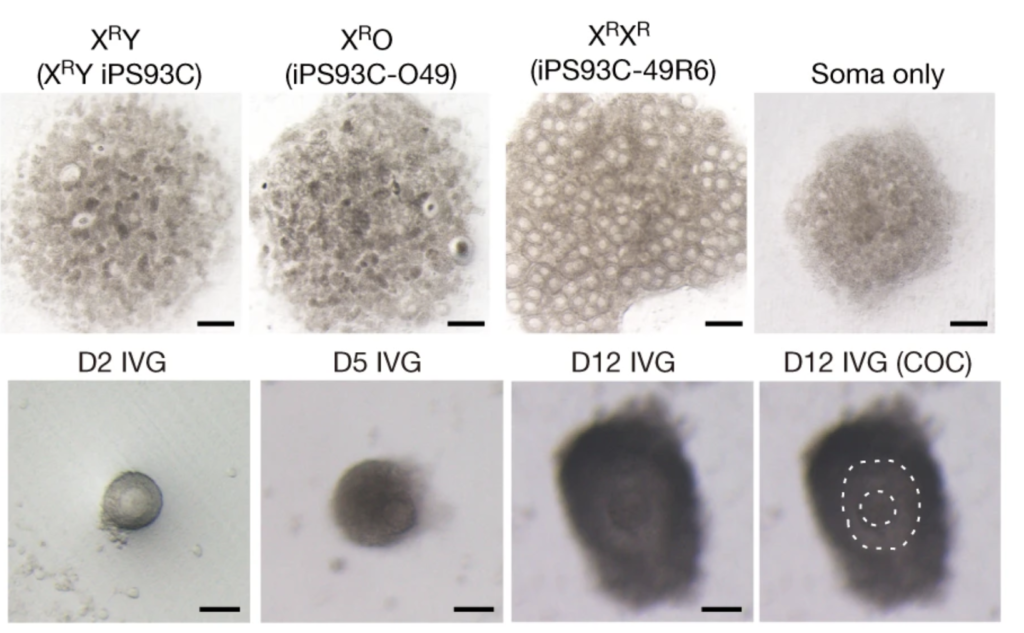
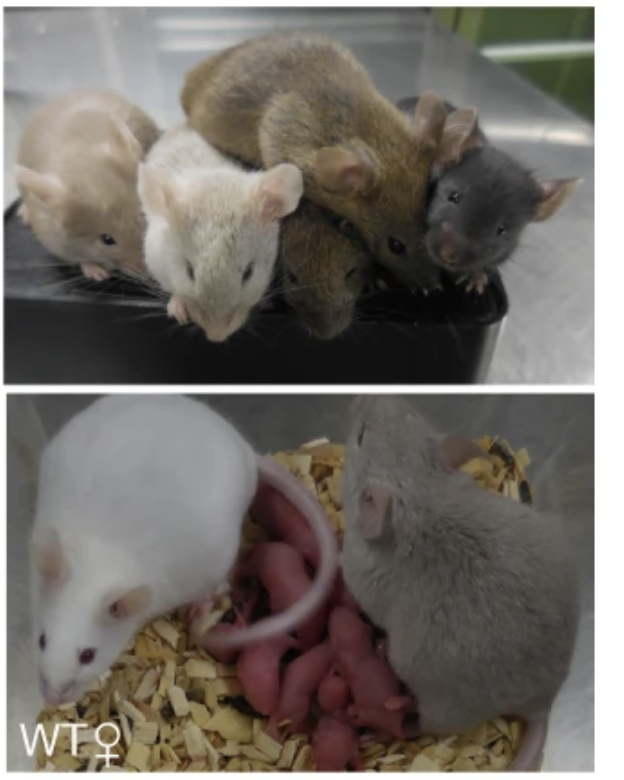
Figure 2. Oocyte differentiation and offspring generated from male iPSc
The study suggests that altering the sex chromosome complement in iPSCs has the potential to address infertility caused by sex chromosome conditions. While it is still far from being applied medically, this methodology provides a valuable tool for understanding the role of sex chromosomes in oogenesis and addressing infertility due to chromosomal or genetic disorders. In addition, the ability to generate functional oocytes from male iPSCs could also have important implications for fertility preservation, as it may allow men with conditions that affect their ability to produce functional sperm to father genetically related children. Finally, this technique can help reduce the reliance on animal models for studying human reproductive biology and development, as in vitro oocytes can provide a model system to elucidate the molecular and cellular mechanisms involved in oocyte maturation, fertilisation, and early embryonic development. This not only promotes ethical and sustainable research practices but also facilitates the development of new reproductive technologies and treatments that can improve human fertility outcomes.
Murakami, K., Hamazaki, N., Hamada, N. et al. Generation of functional oocytes from male mice in vitro. Nature 615, 900–906 (2023). https://doi.org/10.1038/s41586-023-05834-x