 Currently, couples going through In vitro fertilisation (IVF) require the treatment of women with hormones for ovarian stimulation in order to stimulate simultaneously growth of several follicles and to maximize a large number of oocytes available IVF. However, this treatment can have several side-effects and it is not suitable for every woman. This is especially true for women sensitive to the stimulation hormones and women suffering from cancer. Moreover, women who survived cancer usually suffer from infertility and, therefore, can’t benefit from stimulation hormones. One of the options to obtain fully matured oocytes from these patients is to perform the maturation in vitro.
Currently, couples going through In vitro fertilisation (IVF) require the treatment of women with hormones for ovarian stimulation in order to stimulate simultaneously growth of several follicles and to maximize a large number of oocytes available IVF. However, this treatment can have several side-effects and it is not suitable for every woman. This is especially true for women sensitive to the stimulation hormones and women suffering from cancer. Moreover, women who survived cancer usually suffer from infertility and, therefore, can’t benefit from stimulation hormones. One of the options to obtain fully matured oocytes from these patients is to perform the maturation in vitro.
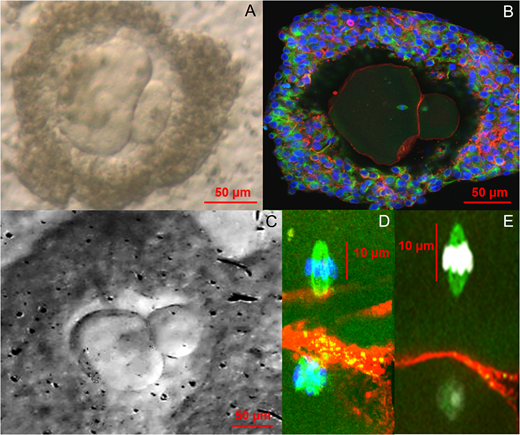
A team of scientists in Edinburgh, lead by Evelyn Telfer, has managed to obtain metaphase II oocytes by in vitro maturation of primordial/unilaminar follicles isolated from human adult ovaries. While this technique has been achieved previously in other mammals, it is the first time that this milestone has been performed using human tissue. This new protocol can push the oocytes to restart meiosis (Metaphase II) and achieve the emission of a polar body in a multi-step serum free culture system.
In this study, samples were obtained from patients undergoing an elective C-section (age 25-39 years old). Small pieces of the ovaries were cultured in different steps for a total of 20-22 days. During step 1, samples were cultured in serum free medium leading to the observation of secondary/multi-laminar follicles. From these, follicles with a diameter of 100-150 μm were selected and further cultured. In step 2, individual follicles were cultured with Activin A for 8 days. This second step resulted in cumulus-oocyte complexes, which, in step 3, were cultured for 4 days in presence of Activin A and FSH. In last step, oocytes with a diameter >100 μm were cultured in SAGE medium to obtain fully matured oocytes with polar bodies (Step 4).
One of the biggest limitations of this study was the low number of samples obtained (n=10) and the low efficiency of the technique. From all the samples, only 87 secondary follicles were obtained from the initial culture. From these, only 9 oocytes developed a polar body at the end of the culture. Moreover, the polar bodies of these 9 oocytes showed an abnormal size.
The researchers did not attempt to fertilise these oocytes to study their potential and the quality of the resulting embryos. However, they plan to fertilise these embryos in the future and study their development in detail.
Similar studies can be expected on the male side. Spermatogonial stem cells could be matured in vitro in order to obtain viable sperm. This technique would be especially relevant for prepubertal boys suffering from cancer and facing infertility due to chemotherapy treatments. Different groups across the world are dedicated to develop this technique. However, the whole process of spermatogenesis has never been fully achieved in vitro in human, mainly due to the difficulties obtaining pre-pubertal tissue. Research in mice has been more successful and some groups have achieved the process by using an organotypic culture system, resulting in fertile offspring. (Sato et al., 2011; Yokonishi et al., 2013).
References:
- McLaughlin, D.F. Albertini, W.H.B. Wallace, R.A. Anderson4, E.E. Telfner. Metaphase II oocytes from human unilaminar follicles grown in a multi- step culture system. Molecular Human reproduction. 2012. Vol.24, No.3 pp. 135–142, 2018. doi:10.1093/molehr/gay002.
- Sato, K. Katagiri, T. A. Gohbara, K. Inoue, N. Ogonuki, A. Ogura, Y. Kubota, T. Ogawa. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2001. 24;471(7339):504-7. doi: 10.1038/nature09850.
- Yokonishi, T. Sato, K. Katagiri, T. Ogawa. In vitro spermatogenesis using an organ culture technique. Methods Molecular Biology. 2013. 927:479-88.

